MD-TEC
The NIHR HRC-DDR is co-located with the Medical Devices Testing and Evaluation Centre (MD-TEC). Initially, set up with part funding from European Regional Development Funds, MD-TEC is a dedicated medical device and technology usability testing facility. Aligned activity alongside the shared oversight of Clinical Director Professor Tom Clutton-Brock means that the NIHR HRC-DDR and MD-TEC are ideally placed to support companies and clinical entrepreneurs navigate the complex journey of health technology development.
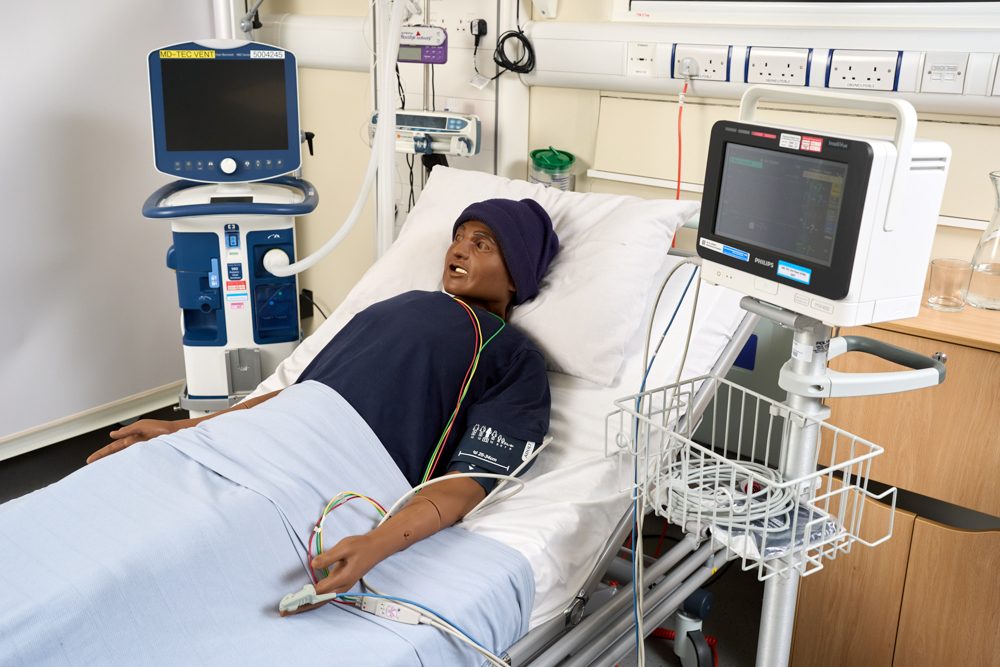
Situated within the Institute of Translational Medicine, MD-TEC is a state-of-the-art facility with a fully equipped operating room. The facility offers the latest in surgical and anaesthetic equipment, a configurable ward area which can be set up as a critical care unit, an emergency department, or a more general ward. There is a large open space which can be configured as an outpatient clinic, physiotherapy unit or a domestic living space. Extensive use is made of HD/4K video recording from multiple angles with remote viewing and streaming capabilities.
Usability testing involves the latest patient simulator technology and phantoms, minimising study requirements. Furthermore, it allows companies the ability to evaluate product iterations whilst avoiding costly design mistakes and condensing the design time frame. Clinical scenarios to assess medical device usability are simulated and involve healthcare professionals specific to the product’s end user and market.